Research Projects
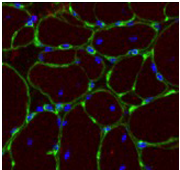
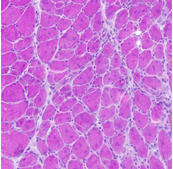
Stem cells are important in the maintenance and repair of many tissues all along the life span. Skeletal muscle presents high plasticity and regenerative properties to keep constant physiological parameters (homeostasis). After a damage, normal skeletal muscle mobilizes tissue-associated endogenous stem cells, mainly satellite cells, to repair damaged myofibers. Indeed, muscle stem cells sustain regeneration that is crucial for muscle homeostasis, as well as the self-renewal mechanisms that maintain their pool constant.
A key issue we address is the tissue environment in which muscle stem cells are activated. Environment plays important roles in the behavior of muscle stem cells and myogenic cells, although the mechanisms are poorly unknown. Various cell types in the vicinity of stem cells communicate with each other to correctly drive regeneration. We explore the roles of immune cells (inflammation), vascular cells (angiogenesis) and interstitial cells (fibrosis) on myogenic cell fate in normal healthy regenerating muscle and during muscular dystrophies. Indeed, myopathies are characterized by the alteration in the environment of muscle stem cells, such as the presence of chronic inflammation and fibrosis, which are detrimental for both tissue repair and cell therapies.
Our goal is to identify the functions for the cell neighbors of muscle stem cells beside their canonical properties and how muscle stem cells are controlled by their closest environment in physiological and pathological contexts. The identification of new molecules or novel functions of already known pathways are the basis for expanding our current understanding about skeletal muscle plasticity and its pathophysiology.
Muscle stem cell neighboring (B. Chazaud)
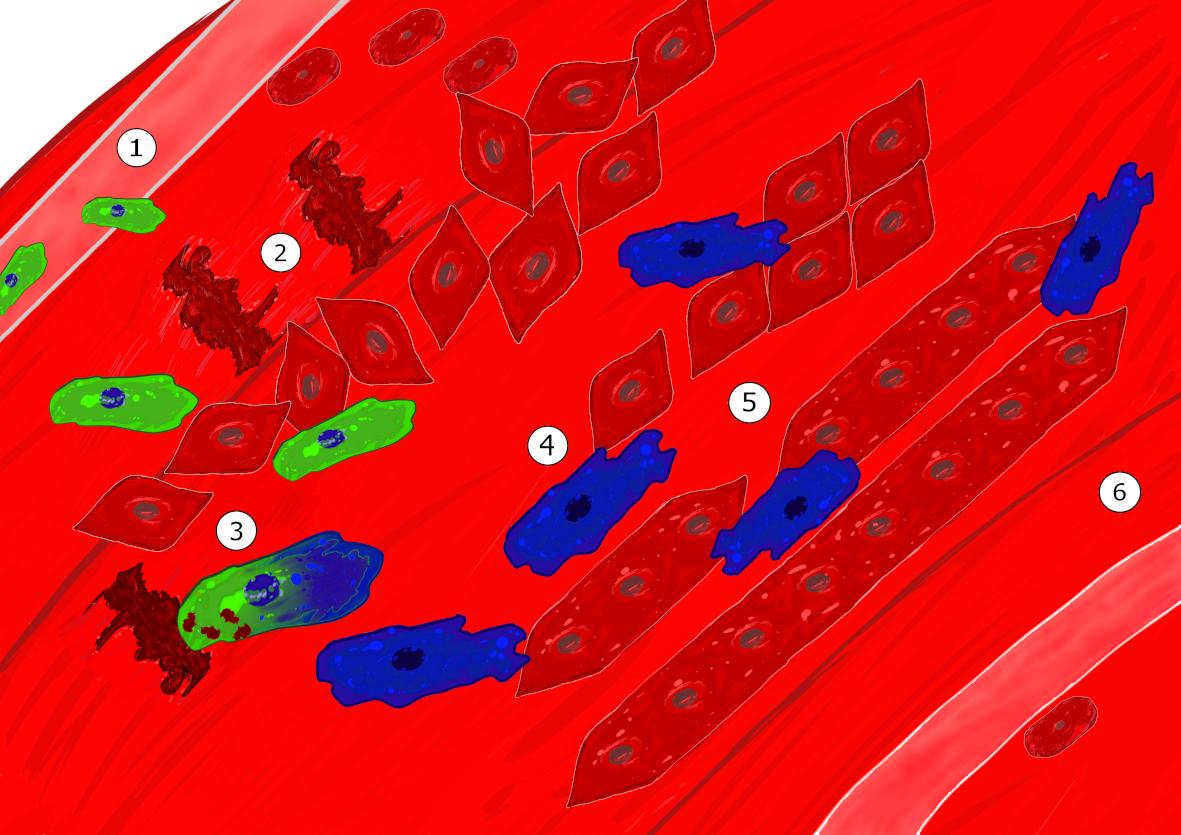
Skeletal muscle regeneration is associated with the presence of macrophages. Two main types of macrophages are present during skeletal muscle regeneration. Soon after injury, inflammatory monocytes enter into the damaged muscle (1) and these inflammatory macrophages stimulate the proliferation of muscle stem cells (2). Upon efferocytosis, which is the engulfment of cell and tissue debris, they switch their phenotype into anti-inflammatory/repair macrophages (3), that sustain myogenic differentiation (4) fusion and myofiber growth (5), as well as angiogenesis and matrix remodeling, until the return to homeostasis (6). Macrophages can be considered as a major actor of the regenerative niche of myogenic cells that helps the sequential steps of skeletal muscle regeneration. An important aspect in macrophage functions is their dynamic during tissue repair, with a crucial moment when the inflammatory phase ends to start the repair process, that is the resolution of inflammation. (©FelixKalfon)
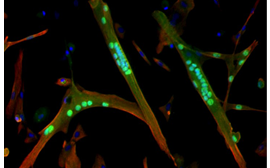
Our research focuses on the molecular mechanisms that regulate the inflammatory status and functions of macrophages during skeletal muscle regeneration, in various physiological an pathological contexts. We also aim at deciphering the cellular and molecular process of the interactions macrophages develop with their neighborhood. This includes myogenic cells, as well as vascular cells and fibro-adipogenic precursors cells (mesenchymal cells involved in tissue remodeling). We work in physiological contexts such as exercise-induced muscle damage and in pathological myopathies where inflammation plays an important role (Duchenne Muscular Dystrophy and Idiopathic Inflammatory Myopathies).
Neuromuscular electrical stimulation training to fight cachexia (J. Gondin)
Cachexia is a common consequence of many chronic diseases including sepsis and cancer. This loss of skeletal muscle mass ultimately results in increased morbidity and mortality. Moreover, treatments, sedation and/or prolonged unloading may further increase muscle deconditioning. On that basis, the development of countermeasures to prevent or attenuate the loss of skeletal muscle mass is of utmost importance for both patients. Our aim is to investigate whether and to what extent NeuroMuscular Electrical Stimulation (NMES) is a promising therapy for increasing force production and promoting muscle growth in preclinical models of cachexia. The program aims at establishing rigorous protocols to exploit the benefit of NMES on muscle function and at identifying the cellular and molecular events underlying those beneficial effects.